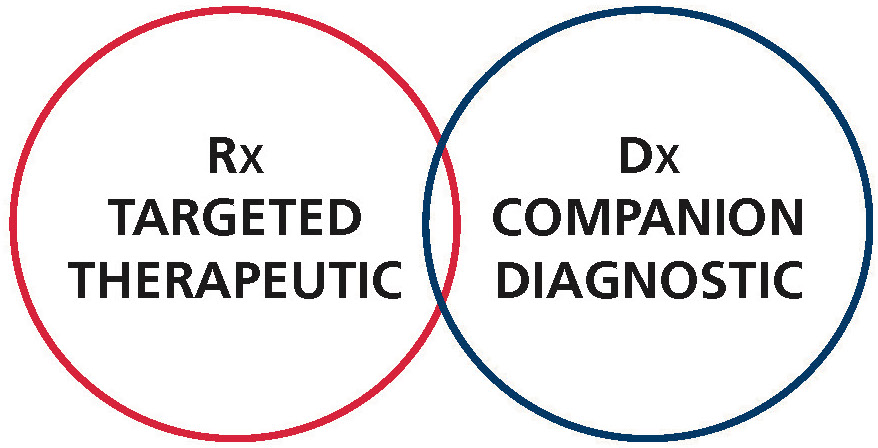
THERANOSTICS – The merging of drug therapy and diagnostics to advance personalised medicine
INTRODUCTION:
In the second issue of our magazine we described the therapeutic use of Iodine, the first true Theranostics compound available. In this edition of our magazine we will put the emphasis on the most recent developments in the utilization of medical isotopes for therapy of cancers.
The modern landmark for Theranostic nuclear medicine originated in the seventies with the discovery of Somatostatin. Somatostatin, a 14.amino acid Cystin bridge-containing peptide, was first discovered in 1973. The elucidation of its three dimensional structure, its metabolism and biological activity site in the following years rapidly lead to the synthesis of a large number of analogs. Identified as the most stable and active in inhibiting the effect of the growth hormone, Octreotide, one of the derivatives, demonstrated enough in vivo stability to obtain regulatory approval in 1988 for the treatment of acromegaly and carcinoid tumors.
The coupling of Octreotide to gamma emitting isotopes in the late 80’s and early 90’s represented a major breakthrough to what we now call molecular targeted imaging. Furthermore it’s labeling with yttrium 90 and lutetium 177 in the early 2000’s started the modern era of theranostic nuclear medicine by introducing the fast growing field of peptide receptor radionuclide therapy (PRRT). In PRRT, specific receptors present at the surface of tumors can now be detected, imaged, treated and followed up with the same peptidomimetic labeled with either imaging or killer isotopes. Labeled with gallium 68, a positron emitter and lutetium 177 a gamma and beta emitter, the somatostatin analog dotatate has recently emerged as a prime tool to diagnose, treat and follow up the treatment’s efficacy of neuroendocrine tumors overexpressing the somatostatin receptor.
Tagged with bifunctional chelating agents, native peptides, hormones, neurotransmitters and peptidomimetics are now emerging as suitable molecules for site-directed targeted imaging and therapy. Among the most promising of these compounds in nuclear medicine are the inhibitors of the prostate specific membrane antigen (PSMA).
PSMA is a cell membrane receptor which is significantly over-expressed in prostate cancers. Its expression increases with tumor aggressiveness, androgen-independence, metastatic disease, and disease recurrence. Evidence suggests that PSMA may perform multiple physiological functions within the cell: a role in signal transduction, cell migration, receptor function for an unidentified ligand and nutrient uptake such as glutamate and folate have been suggested.
Having a sensitive and specific biomarker to localize primary and metastatic prostate cancer would greatly improve the algorithm for the diagnosis and management of prostate cancer. Other than skin cancer, prostate cancer is the most common cancer in North America. About one out of seven men in the US will be diagnosed from prostate cancer during his lifetime.
Since 2012, the number of PSMA clinical studies using has exponentially increased. Among these agents, the 68Ga- and 18F-labeled compounds have attracted the most attention, as these compounds can be used for PET/CT imaging. However, the availability of 123I or 99mTc also will allow SPECT/CT imaging in centers without facilities for PET.
Based on these studies, the promising uses of imaging with labeled PSMA ligands in the management of prostate carcinoma include: the primary staging of high risk cancer disease, the biochemical recurrence with low PSA levels (as low as 0.2 ng/ml), identification of lesions for biopsy targeting after negative previous biopsy, the monitoring of systemic treatment in metastatic disease, the active surveillance and the treatment monitoring after 177Lu-PSMA ligand therapy.



