Positron Emission Tomography (PET)
Positron Emission Tomography (PET) is a cutting edge, non-invasive, diagnostic imaging technique which allows the measurement of biochemical processes or the expression of cellular receptors by the use of positron-emitting radioactive tracers. The imaging tracers most often contain atoms naturally found in organic molecules, but in the form of radioactive analogues of Oxygen (15O), Nitrogen (13N), Carbon (11C) or Fluorine (18F) atoms.
Developed during the 70s to study the normal and pathological brain function, in the 90s, PET became an important clinical tool for oncological imaging following the demonstration of its usefulness in the detection of several cancer types.
Initially confined to research centers, PET has spread rapidly since 1998 to the vast majority of important oncologic centers in industrialized countries. Since 2001, PET has been paired to axial computed tomography (CT) (Figure 1) in order to better locate lesions by relation to the anatomical structures. It also facilitates the interpretation of results by improving the specificity and, furthermore, combined PET/CT data allows the planning of radiation therapy treatment. PET/CT images also have a considerable advantage for planning the surgical approach, since an accurate anatomical localization may be established. It is also possible to merge any PET study to any three-dimensional imaging including MRI and SPECT tomography, even if they have been obtained on different devices or at different times (Figure 2).
Gradually, the price of PET/CT devices has dropped by half and now can be purchased for 1.2M$. The scanner allows the accomplishment of a full study from the neck to the pelvis in less than 25 minutes, thus improving considerably the productivity of PET and helping to reduce the cost of each exam (throughput of 15-20 exams per scanner per 8 hours). Since 2005, PET/CT technology deployed quickly in the public and the private health systems across Canada. However, in 2014, access to PET is very different from east to west provinces and Canada is still behind many developed countries. Quebec is by far the province providing the best access to this technology. Quebec is also the first province which has deployed the latest generation of PET/CT devices to regional hospitals away from academic and research centers.
As with the studies performed in conventional nuclear medicine (bone or thyroid scan, myocardial perfusion, etc.), PET/CT is performed after the intravenous injection of a radioactive substance called radiotracer. Over the past few years, several radiotracers have been developed for the detection of cancer and the most important is the fluorine-18 Fluorodeoxyglucose (18F-FDG). The fluorine-18, with a half-life of 110 minutes, is the isotope of choice for cancer imaging.  This isotope can easily be distributed to multiple institutions and its long half-life allows it to accumulate at levels that are sufficient for imaging with adequate contrast for tumor detection. The molecule of 18F-FDG is an analogue of glucose obtained by substituting a hydroxyl (OH) group of a glucose molecule by a radioactive fluorine atom with a nucleus that contains more protons than neutrons. This atypical ratio of protons/neutrons makes the atom unstable and the latter must expel a positive charge in the form of a positron to regain its stability. The PET devices are conceived to detect radioactive emissions induced by these positrons, and to precisely locate this emission inside the body. At the cellular level, the 18F-FDG uses the same transmembrane carriers as glucose, and its passage is transporter – and insulin – dependent. After its entry into the cell, the 18F-FDG is phosphorylated by the hexokinase, but it rapidly stops advancing into the glycolysis cascade. It thus becomes sequestered in the cell where it accumulates. The 18F-FDG permits to obtain cellular information relative to the cell viability and metabolism based on the metabolic rate of the cellular glucose.
This isotope can easily be distributed to multiple institutions and its long half-life allows it to accumulate at levels that are sufficient for imaging with adequate contrast for tumor detection. The molecule of 18F-FDG is an analogue of glucose obtained by substituting a hydroxyl (OH) group of a glucose molecule by a radioactive fluorine atom with a nucleus that contains more protons than neutrons. This atypical ratio of protons/neutrons makes the atom unstable and the latter must expel a positive charge in the form of a positron to regain its stability. The PET devices are conceived to detect radioactive emissions induced by these positrons, and to precisely locate this emission inside the body. At the cellular level, the 18F-FDG uses the same transmembrane carriers as glucose, and its passage is transporter – and insulin – dependent. After its entry into the cell, the 18F-FDG is phosphorylated by the hexokinase, but it rapidly stops advancing into the glycolysis cascade. It thus becomes sequestered in the cell where it accumulates. The 18F-FDG permits to obtain cellular information relative to the cell viability and metabolism based on the metabolic rate of the cellular glucose.
The excretion of 18F-FDG is mainly through the urinary tract, regardless of if the patient is diabetic or not, because the 18F-FDG molecule is not completely reabsorbed by the renal tubules, unlike glucose. There is also a certain proportion, very variable from one individual to another, which is excreted by the intestines. Figure 3 (left) shows a normal biodistribution of 18F-FDG compared to the image on the right which illustrates the important consumption of 18F-FDG by striated muscle due to a non-respected fasting/ glucose-free procedure. Figure 3 (right) is considered non-diagnostic. Consequently, the exam must be repeated at a later date.
The 18F-FDG PET is aimed at four main fields of clinical application: oncology, cardiology (Figure 4), neurology (Figure 5) and infection/ inflammation (Figure 6). Overall, in a general hospital, more than 95 per cent of the examinations currently carried out are for oncologic indications. The rationale behind the use of 18F-FDG PET in oncology is based on the increased use of glucose by the neoplastic cells, a phenomenon closely linked to the neoplastic transformation. A rapidly growing neoplasia is also ischemic in its center, hence favoring the metabolic pathway of lactic acid, which greatly increases the demand for glucose. A non-negligible proportion of the uptake also comes from the inflammatory cells surrounding the tumor. It should be noted, however, that these phenomena vary significantly depending on the type of neoplasia.
Although PET is excellent to detect neoplastic lesions, it has limitations. Some cancers have slower growth and do not substantially increase their 18FFDG accumulation and may remain undetectable (false negative). The activated neutrophils and macrophages can consume a lot of glucose and the highly inflammatory lesions can also incorporate this radiotracer (false positive). In particular, active granulomatous inflammation (tuberculosis, sarcoidosis) as well as abscesses can cause false positive results (Figure 7). Conversely, some well-differentiated cancers, such as prostate cancer, high mucinous content tumours, well differentiated neuroendocrine tumors, and certain lobular breast cancers may have a low uptake. Others, such as the hepatocarcinoma, possess phosphorylases, which allow cells to quickly eliminate the 18F-FDG.
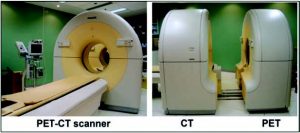 Figure 1: PET/CT scanner allowing to sequentially obtain an axial tomography (CT) and a positron emission tomography (PET) during a same medical visit.
Figure 1: PET/CT scanner allowing to sequentially obtain an axial tomography (CT) and a positron emission tomography (PET) during a same medical visit.
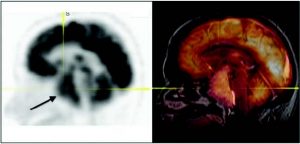 Figure 2: Current software which enables the merging of PET not only with the CT, but with all 3D imaging modalities, including MRI. PET-MRI fusion allows better localization and characterization of the neoplastic cerebral lesions (the arrow shows a voluminous pituitary prolactinoma).
Figure 2: Current software which enables the merging of PET not only with the CT, but with all 3D imaging modalities, including MRI. PET-MRI fusion allows better localization and characterization of the neoplastic cerebral lesions (the arrow shows a voluminous pituitary prolactinoma).
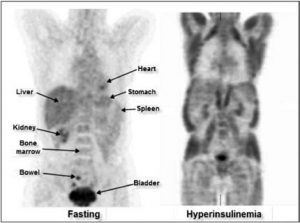
Figure 3: PET studies performed in two different patients. The image on the left illustrates a normal biodistribution of FDG in a patient having observed the fasting procedure. The image on the right has been obtained in a patient with dextrose solute (diffuse muscle uptake induced by hyperinsulinemia).
A list of clinical indications for oncology PET with 18F-FDG is detailed in Tables 1 and 2. Taken together, lung cancer (Figure 8) and lymphoma indications represent about 50% of the available imaging time on a PET scanner.
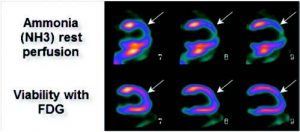 Figure 4: This PET exam uses ammonium to assess myocardial perfusion at rest and FDG for viability. This exam is more sensitive than Thallium for the demonstration of severely ischemic regions at rest or hibernating myocardium in order to orient the therapeutic approach (to increase the ejection fraction and decrease morbidity). The example illustrates severe hypoperfusion at rest (rest ischemia) in the region of the descending anterior, completely viable on the FDG study. The study therefore suggests that this wall will resume a normal kinetic after revascularisation that will result in gain of ejection fraction.
Figure 4: This PET exam uses ammonium to assess myocardial perfusion at rest and FDG for viability. This exam is more sensitive than Thallium for the demonstration of severely ischemic regions at rest or hibernating myocardium in order to orient the therapeutic approach (to increase the ejection fraction and decrease morbidity). The example illustrates severe hypoperfusion at rest (rest ischemia) in the region of the descending anterior, completely viable on the FDG study. The study therefore suggests that this wall will resume a normal kinetic after revascularisation that will result in gain of ejection fraction.
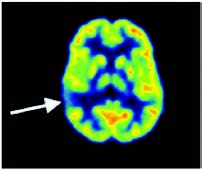 Figure 5: Brain FDG PET in search of inter-ictal epilepsy foyers. Right temporal hypometabolism testifies to an epileptic foyer (arrow).
Figure 5: Brain FDG PET in search of inter-ictal epilepsy foyers. Right temporal hypometabolism testifies to an epileptic foyer (arrow).
PET should be an accessible exam to be considered by a specialist as well as the family physician. However, for a better management of the resource, it is important to recognize the strengths and weaknesses of the technique in order to ensure that the examination can answer the clinical and therapeutic dilemma. The following guiding points are to be considered if a PET/CT exam is to be requested:
Does the patient need to be fasting? Is fasting mandatory for all patients? What about diabetic patients?
Patients arriving for their exam must obey the fasting procedure (sugar-free) for at least 6 hours prior to the exam. Diabetic patients can take their oral hypoglycemic agents and their slow onset insulin dose in the morning of the examination day. However, special attention should be paid to metformin because this medication is responsible for a very intense bowel uptake (Figure 9). Thus, metformin should be stopped at least two days prior to PET if an intestinal lesion is suspected.
All sources of glucose (including lozenges, mints, gums, glucose in solution) must be strictly avoided so as to maintain circulating insulin at the basal level. If there is any doubt about the patient’s glucose intake in the last six hours, the study should be deferred in order to observe fasting procedure for at least six hours (Figure 3). It is also required that the capillary blood glucose level at the time of injection is less than 10 mmol/L. If the result is higher than 10 mmol/L, one to two doses of rapid i.v. insulin can be injected in order to normalize the blood glucose level. An additional waiting period of 60 minutes is necessary to allow the exogenous insulin to be metabolized. The exam will be postponed to a later date if it is not possible to normalize the blood glucose level. It is therefore important to ensure that the diabetes is well controlled and that the glucose level is normalized (or almost) and stable before requesting an 18F-FDG study.
Is there a reasonable doubt of neoplasia in respect to the clinical and para-clinical evaluation?
The prescription of an oncology PET study should ideally be limited to patients with proven or strongly suspected neoplasia. A clear clinical question should be included in the request form for the exam. It is the responsibility of the requesting physician to be as clear as possible.
What is the location of the neoplasia?
The degree of metabolism of a tumor must be put in relationship with the basal metabolism of the organ in which the tumor is sought (Figures 2 and 3). In some cases, this can cause interpretation problems and can limit the sensitivity of the examination. The bladder comes in at the first place of hypermetabolic organs due to the physiological urinary excretion. Fortunately, it is easy to significantly decrease the physiological activity in urinary bladder by diluting its activity through the i.v. administration of furosemide. This protocol allows therefore to image high grade bladder cancer and to make the assessment of its extension (Figure 10). In contrast, since this is not a procedure carried out on all patients, it is important to specify in the request for examination if there is a suspicion of bladder neoplasia, lesion to the outer wall (implant of a gynecological neoplasia) or at the edge of the bladder (metastatic lymph node). The brain comes in second place of hypermetabolic organs. Unlike the bladder, there is no easy method to decrease brain activity other than by sedation. It is therefore difficult to locate brain metastases in this physiologically very active organ. MRI remains the imaging of choice in the assessment of neoplastic cerebral lesions, but PET is the imaging of choice for monitoring post radiotherapy response.
What is the size of the lesion under evaluation?
The sensitivity and resolution of PET equipment is increasing from year to year. In the optimal conditions, the technology currently available can detect lesions in the vicinity of 4.5 mm. Unfortunately, these perfect conditions are almost impossible to obtain in the human body, and a reasonable estimate of the camera resolution would be at about 6 mm. If the anomaly to be imaged is sought in a mobile organ, the sensitivity decreases as a function of the amplitude of the movement. It is therefore difficult to identify a nodule of 6 mm located at the base of the lung or an infra- centimeter hepatic metastasis juxtaposed to the diaphragmatic dome. To overcome this limit, imaging techniques synchronized with the respiratory rate are now available (respiratory gating).
What is the histological type of the initial tumor and its grade in a context of staging, restaging or when evaluating treatment response?
The higher or more undifferentiated histological the grade is, the more it will accumulate 18F-FDG. Because of their low glucose metabolism, some histological types do not uptake any or will accumulate very little of 18F-FDG so that the role of PET with FDG is limited for these types of tumors: well differentiated prostate cancer, some hypernephroma, small lymphocytic lymphoma (chronic lymphoid leukemia), marginal zone lymphoma, lymphoplasmocytary lymphoma (Waldenstrom), leukemia, well differentiated hepatocellular carcinoma, minimally invasive lung carcinoma (bronchiolo-alveolar), any tumors with high mucin component, low grade neuroendocrine tumor, low grade sarcomas (particularly liposarcoma), teratoma, and some well differentiated breast cancer (particularly the lobular carcinoma).
How much time should we wait between a PET study and the last surgery, radiotherapy or chemotherapy?
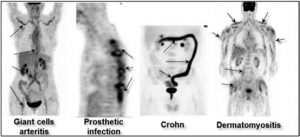 Figure 6: PET FDG is not only useful in oncology. It can be used for the diagnosis and monitoring of giant cell arteritis, the search for infectious foyers (ex: infection of orthopaedic materials), the diagnosis of myositis/dermatomyositis and even in the assessment of inflammatory intestinal diseases.
Figure 6: PET FDG is not only useful in oncology. It can be used for the diagnosis and monitoring of giant cell arteritis, the search for infectious foyers (ex: infection of orthopaedic materials), the diagnosis of myositis/dermatomyositis and even in the assessment of inflammatory intestinal diseases.
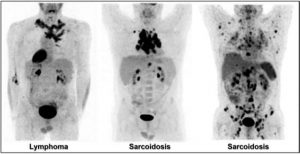 Figure 7: Active chronic inflammatory granulomatosis conditions, like sarcoidosis, can resemble a lymphoma (center), or even a plurimetastatic disease (right). Some criteria, including the symmetric hilum distribution, the disproportion between the size of lymph nodes and their activity, the presence of lymph nodes and splenic calcifications, are elements in favor of a granulomatous disease. In cases where presentation is more atypical (image on the right), only a biopsy can differentiate between the two entities.
Figure 7: Active chronic inflammatory granulomatosis conditions, like sarcoidosis, can resemble a lymphoma (center), or even a plurimetastatic disease (right). Some criteria, including the symmetric hilum distribution, the disproportion between the size of lymph nodes and their activity, the presence of lymph nodes and splenic calcifications, are elements in favor of a granulomatous disease. In cases where presentation is more atypical (image on the right), only a biopsy can differentiate between the two entities.
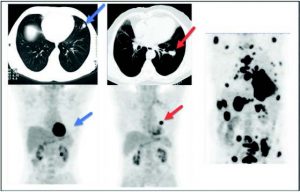 Figure 8: Evaluation of an undetermined lung nodule. Left Images (CT and PET): Lung nodule to the lingula presenting no significant metabolic activity, compatible with a benign/inflammatory nodule (blue arrows). Center Images (CT and PET): Lung Nodule near the left hilum presenting very significant metabolic activity that is compatible with primary pulmonary malignancy (red arrows). Right Image (PET alone): Plurimetastatic disease (bone, lymph nodes, adrenal glands) from the left lung.
Figure 8: Evaluation of an undetermined lung nodule. Left Images (CT and PET): Lung nodule to the lingula presenting no significant metabolic activity, compatible with a benign/inflammatory nodule (blue arrows). Center Images (CT and PET): Lung Nodule near the left hilum presenting very significant metabolic activity that is compatible with primary pulmonary malignancy (red arrows). Right Image (PET alone): Plurimetastatic disease (bone, lymph nodes, adrenal glands) from the left lung.
Especially for the evaluation of a local recurrence or that of a treatment response, it is recommended to wait four weeks between the PET study and surgery or chemotherapy, or to wait three months after the last radiation treatment, since the local residual inflammation could cause false positive results. If performed too soon post-treatment, the evaluation may be associated, depending on the case, to a higher rate of false positives or false negatives. It is also important to mention that if the patient is under hormonal therapy, this medication can also affect PET results, same as chemotherapy.
Is there an infection near the site under evaluation? Is the patient known for a non-neoplastic disease that naturally uptakes 18F-FDG?
FDG PET does not provide a way to differentiate between a neoplastic lesion and an active infectious or inflammatory process (Figure 6), since the two latter will avidly capture the 18F-FDG. It is therefore difficult, for example, to differentiate active tuberculosis foyer from a primary pulmonary malignancy or an abdominal abscess of a colonic neoplasia from a lymphoma. Some benign pathologies, like sarcoidosis, active Wegener, tuberculosis, uterine fibroid, thyroiditis, stomach ulcers, acute or chronic cholecystitis and many others may capture FDG. Without biopsy of the lymph node, it is often difficult to differentiate by PET a sarcoidosis from a metastasis or a lymphoma (Figure 7), a uterine fibroid tumor from a sarcoma or yet, the histiocytosis from a multifocal bone metastasis.
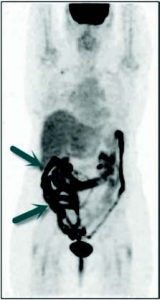 Figure 9: FDG PET performed while taking metformin. Metformin modulates a highly intense FDG accumulation in the bowel (blue arrows) which makes it impossible to detect bowel lesions. Metformin should be stopped at least two days before FDG PET.
Figure 9: FDG PET performed while taking metformin. Metformin modulates a highly intense FDG accumulation in the bowel (blue arrows) which makes it impossible to detect bowel lesions. Metformin should be stopped at least two days before FDG PET.
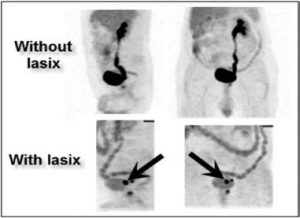 Figure 10:In the physiologically hypermetabolic organs, some maneuvers may be attempted in order to decrease the basal metabolism and to allow visualization of the tumor. Bladder cancer is the typical example of how the use of a diuretic helps the bladder to drain quickly so that the tumor can be easily set apart (black arrows).
Figure 10:In the physiologically hypermetabolic organs, some maneuvers may be attempted in order to decrease the basal metabolism and to allow visualization of the tumor. Bladder cancer is the typical example of how the use of a diuretic helps the bladder to drain quickly so that the tumor can be easily set apart (black arrows).
In other words, FDG may accumulate in places with active inflammation, be it acute, chronic, infectious or granulomatous. The distribution of the radiotracer and the appearance of lesions can sometimes allow the nuclear medicine specialist to distinguish between an infection, an inflammatory process or a neoplastic lesion, but it is important to remember that these conditions represent the most frequent cause of false-positive results.
FDG PET is now the oncology standard for several types of cancers. This very powerful diagnostic tool is only in its early stages in Canada and will be called upon even more in the coming years. Even if FDG is an excellent radiotracer for tumors and metastases localization, some cancers cannot be easily assessed with this radiotracer. Consequently, there is a need for new clinical tracers to increase the diagnostic accuracy of PET for cancers where FDG is less efficient. Sodium fluoride is one of the new tracers in clinic which allow earlier detection of the bone metastases (Figure 11). 18F-MFES, an oestrogen derivative under clinical trial in BC and Quebec, is one of the most promising tracers for the detection and staging of hormonosensitive breast cancer (Figure 12).
Since there are multiple factors to consider in a FDG PET study, it is crucial to provide maximal clinical information to the nuclear medicine specialist who will be interpreting the imaging results (pathology reports, summary of surgical procedures, radiology results, blood biochemistry) in order to give a more precise answer to the clinical question. And,for more complex cases, a discussion with the nuclear medicine specialist may be relevant before prescribing the exam.
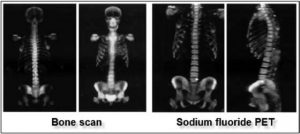 Figure 11: The future of the PET lays within the development of new radiotracers enabling sensitivity increase of already existing exams and/or newer indications. The development of sodium fluoride PET (NaF PET) as a replacement for the conventional bone scan is an example. This study is carried out 30 to 45 minutes after the injection of the radiotracer and the acquisition of the images only lasts for 35 minutes (compared to a waiting time of 4 hours and 40 minutes imaging, on average, for a regular bone scan). With NaF PET, which is more sensitive and faster, it is possible to locate metastases as small as 5 mm.
Figure 11: The future of the PET lays within the development of new radiotracers enabling sensitivity increase of already existing exams and/or newer indications. The development of sodium fluoride PET (NaF PET) as a replacement for the conventional bone scan is an example. This study is carried out 30 to 45 minutes after the injection of the radiotracer and the acquisition of the images only lasts for 35 minutes (compared to a waiting time of 4 hours and 40 minutes imaging, on average, for a regular bone scan). With NaF PET, which is more sensitive and faster, it is possible to locate metastases as small as 5 mm.

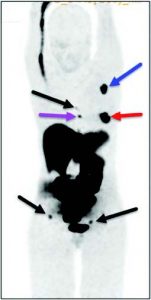 Figure 12: PET performed with 18F-MFES, an oestrogen derivative highly sensitive to detect hormonosensitive breast cancer and metastasis. Clinical trials, funded by the Canadian Breast Cancer Foundation, are underway in Quebec and in the initial phase in BC. Red arrow: Primary breast cancer. Blue arrow: axillary metastasis. Purple arrow: internal mammary metastatic lymph node. Black arrows: Bone metastasis.
Figure 12: PET performed with 18F-MFES, an oestrogen derivative highly sensitive to detect hormonosensitive breast cancer and metastasis. Clinical trials, funded by the Canadian Breast Cancer Foundation, are underway in Quebec and in the initial phase in BC. Red arrow: Primary breast cancer. Blue arrow: axillary metastasis. Purple arrow: internal mammary metastatic lymph node. Black arrows: Bone metastasis.

