Theranostics

Dr. Kamel Hasan, Olfat, MBChB, FRCR, FRCPC
Nuclear Medicine and Molecular Imaging Physician
Hamilton Health Sciences and St. Joseph’s Healthcare McMaster University
Ontario, Canada
INTRODUCTION
Over the past 30 years, there have been colossal advances in the way we have lived life through the development and innovation of scientists worldwide. From the creation of the internet, to the development of the smart phone, accessible Wi-Fi, and more recently to self-driving cars and smart home systems. When people think of the word technology, Steve Jobs’s Apple devices, or Elon Musk’s Tesla comes to mind. Although healthcare is not the first field that pops into people’s minds when innovation and technology are asked about, healthcare advances are important to consider. In 1950, the global average life expectancy was 46 years; in contrast, the WHO reports that the global life expectancy in 2019 is 73.4 year. This 27-year increase in life expectancy can be attributed to the development of our healthcare system and the newer therapies that are available.
Within healthcare, cancer has been one of the longest studied diseases. It is well known that cancer is one of the most important health problems that we face as it is a leading cause of death worldwide, accounting for nearly 10 million deaths per year, or nearly one in six deaths. As much as cancer prevention is important, finding new scientific ways to treat this nasty disease has been a goal for many top researchers all over the world over many decades now. This article will briefly explain a relatively new smart way of cancer treatment that has the potential of being applied to many types of cancer in different age groups. This concept is called theranostics.
WHAT IS CANCER?
Cancer is a generic term for a large group of diseases that can affect both sexes at different ages and may affect any part of your body. It is caused by the transformation of normal body cells into tumour cells. These usually arise from random unpredictable DNA mistakes that happen on a cellular level. These mistakes, or what we call mutations, lead to unregulated cell division and much faster growth compared to normal cells associated with cell death inhibition. Eventually, this leads to the formation of large masses that replaces normal body tissue. This causes disruption of the normal function of the organs they occupy, which leads to multiple complications and, if untreated, death.
It is believed that the cause of cancer is multifactorial where genetic factors play an important role, as well as environmental exposure to certain agents that we call carcinogens. These could be chemical (tobacco and alcohol for example), physical (like ultraviolet and ionizing radiation), and biological (like infections).
RADIONUCLIDIC THERAPY
The main known therapeutic options for cancer are: surgery, drug therapy (including chemotherapy, immunotherapy and hormonal therapy), and radiation therapy. Radiation therapy uses beams of intense energy to kill cancer cells by destroying the genetic material (DNA) that controls how cells grow and divide. Radiation kills healthy cells as well as cancer cells, but cancer cells are easier to kill because they are dividing faster.
External radiation therapy is the most common type of radiation therapy to treat cancer. It uses external radiation aiming at specific organ or part of the body where the cancer is. The main disadvantage is the direct damage to the surrounding tissue as it is not specifically targeting the cancer cells and all the cells in the path of radiation will be affected.
Another radiation-based treatment option available for certain types of cancer is the use of targeted radionuclide therapy, where a radioactive substance is administered to patients. This type of therapy is a systemic treatment, reaching cells throughout the body by travelling through the bloodstream. However, it is smart enough to target mainly the tumor cells, unlike chemotherapy which targets all rapidly dividing cells leading to many undesirable side effects.
Targeting cancer cells specifically can be achieved by using a specific molecule that would target a specific receptor that is found on the membranes of cancer cells. The tumor cells in this case are rather more differentiated and have partially preserved the characteristics of the original cells that they develop from. The key molecule that is usually injected into the patient has an accessary radioactive isotope attached to it which emits radiation that can be imaged to localize the tumor cells, and/or to destroy these cancer cells. Of all the disciplines in medicine, nuclear medicine is the best suited to achieve these goals.
Different types of radioactive isotopes can be used depending on whether these are needed for diagnosis to localize the tumor; or for therapy where more intense and more damaging effect is required to kill the cancer cells. This selectivity in targeting the tumor cells will significantly reduce the undesirable collateral damage of normal tissue that is difficult to avoid with other types of radiation or chemotherapy.
THE CONCEPT OF THERANOSTICS
The word “Theranostics” is a combination of two words: Therapy and Diagnosis. It is a transition from conventional therapy to a contemporary, personalized, and precise approach in cancer therapy. It combines specific targeted therapy based on specific targeted diagnostic tests improving both patients’ outcome and safety at the same time. In other words, using a specific key molecule attached to a diagnostic radioisotope to detect and assess the phenotype of the cancer (Diagnosis), and if positive, then use a similar key molecule attached to a therapeutic more damaging radioactive isotope to selectively damage the cancer cells (Therapy). Consequently, this ensures that the radioactivity will target the desirable sites of tumor spread. It is important to monitor the effects and response to this smart therapy, and for that we can use the diagnostic functional imaging repeatedly. A distinctive advantage of molecular targeted radiotherapy is having the radiation dose distributions available before initiating the therapy for a patient. This can be used to select the preferred drug at the appropriate dose for a patient, thereby maximizing the desirable tumor damaging effect and minimizing the undesirable potential side effects.
It is the nature of cancer cells to gain aggressiveness and build resistance to different types of therapies overtime. It is well known that different clones of the same cancer can co-exist in the same patient with  variable levels of aggression. This heterogeneity of tumor cells is an important factor that will affect prognosis and overall survival. Nuclear medicine and molecular imaging can play an important role in detecting this heterogeneity and further characterization by combing different tumor targeted agents, for example adding radioactive glucose F18- FDG imaging which can detect the most aggressive clones of cancer within the body. This will help guiding management and plan future therapies that will target the most aggressive phenotype and eventually prolong survival and improve quality of life.
variable levels of aggression. This heterogeneity of tumor cells is an important factor that will affect prognosis and overall survival. Nuclear medicine and molecular imaging can play an important role in detecting this heterogeneity and further characterization by combing different tumor targeted agents, for example adding radioactive glucose F18- FDG imaging which can detect the most aggressive clones of cancer within the body. This will help guiding management and plan future therapies that will target the most aggressive phenotype and eventually prolong survival and improve quality of life.
EXAMPLES OF THERANOSTICS
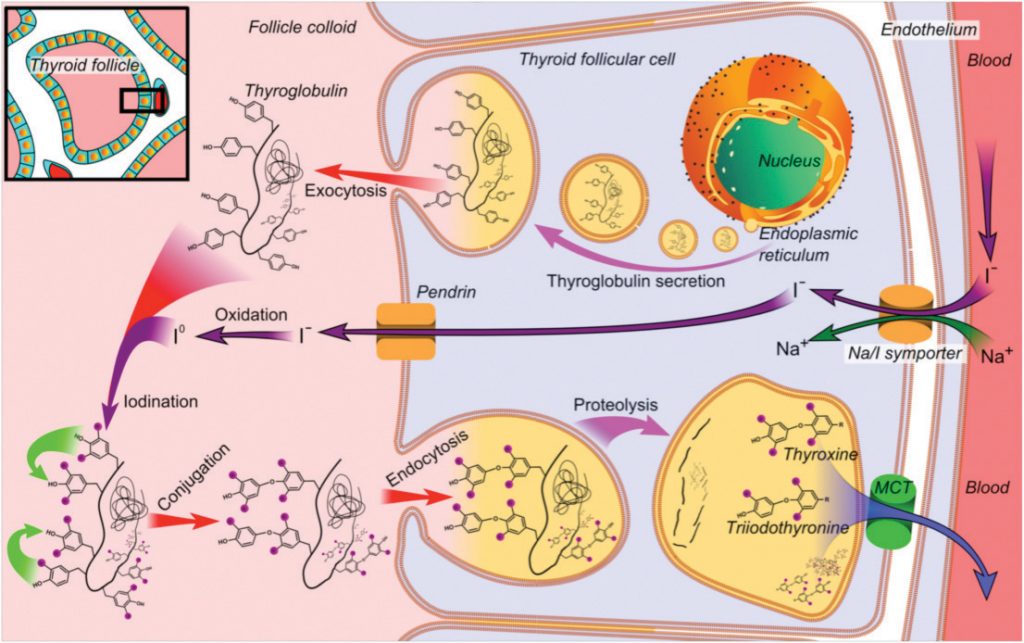
IODINE THERAPY FOR THYROID DISEASE
Radioactive iodine (RAI) is known to be the first theranostic agent that is still considered an essential component of thyroid disease management. It is used to treat both hyperthyroidism and differentiated thyroid cancer (DTC). Iodine-130 and I-131 were first employed to treat thyrotoxicosis in 1941, and thyroid cancer in 1943. The RAI treatment of DTC is based on the principle of the sodium-iodide symporter that is expressed on the differentiated thyroid cancer cells and have the ability of trapping circulating RAI. As such, it is also effective in the treatment of residual and metastatic disease.
PEPTIDE RECEPTOR RADIONUCLIDIC THERAPY (PRRT) FOR NEURO ENDOCRINE TUMORS (NET)
Neuroendocrine neoplasms represent a diverse group of tumors which most commonly arise from gastroenteropancreatic structures. Although neuroendocrine tumors are considered rare, there is a global rise in incidence. The relatively differentiated nature of the disease, with specific expression of somatostatin receptors (SSTR), and its indolent course, make this disease perfect to be targeted by peptide receptor radionuclidic therapy (PRRT) using therapeutic radionuclides (beta- or alpha-emitting radioisotopes). PRRT binds to those receptors on the tumor cells and destroys them with radioactivity.
 It is by the FDA in early 2022. This targeted therapy appears to be the first of many future agents that will help fulfil a major clinical need in the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC). not a cure, but PRRT can effectively slow or stop tumor growth. This can be achieved by applying the concept of theranostics and assess the receptor status of the tumor cell before the actual therapy utilizing positron emission tomography/computed tomography (PET/CT) using Ga-68-labeled somatostatin analogs. This diagnostic tracer binds specifically to those somatostatin receptors and allow the molecular imaging and characterization of NETs with a very high sensitivity and specificity for early identification of metastases.
It is by the FDA in early 2022. This targeted therapy appears to be the first of many future agents that will help fulfil a major clinical need in the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC). not a cure, but PRRT can effectively slow or stop tumor growth. This can be achieved by applying the concept of theranostics and assess the receptor status of the tumor cell before the actual therapy utilizing positron emission tomography/computed tomography (PET/CT) using Ga-68-labeled somatostatin analogs. This diagnostic tracer binds specifically to those somatostatin receptors and allow the molecular imaging and characterization of NETs with a very high sensitivity and specificity for early identification of metastases.
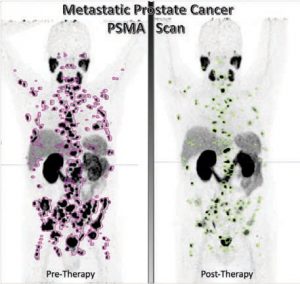
PROSTATE SPECIFIC MEMBRANE ANTIGEN (PSMA) TARGETED THERAPY FOR PROSTATE CANCER
Prostate cancer is the second most frequently diagnosed cancer in men worldwide and the fifth leading cause of cancer caused death. Prostate-specific membrane antigen (PSMA) is expressed on the cell surface in normal prostate tissue and is overexpressed in prostate cancer by several orders of magnitude. Targeted radionuclide therapy with prostate-specific membrane antigen (PSMA)-targeting ligands is a novel therapy that revolutionized the role of nuclear medicine in prostate cancer management. Both lutetium-177 PSMA, the therapeutic agent, and gallium-68 PSMA, the diagnostic agent, were approved
Interestingly, PSMA-targeted approaches are expected to move into earlier mCRPC and mHSPC (metastatic hormone sensitive prostate cancer), due the frequent expression of PSMA in early disease, in addition to the acceptable safety profile of this therapy. PSMA-targeted therapy proved to significantly improve prostate cancer survival rates and quality of life, as well as extend the time it takes for the disease to progress.
FUTURE DIRECTIONS
Targeted radionuclide therapy is an excellent example of theranostics. Molecular imaging and therapy are part of a paradigm shift to individualize or personalize patient care. Radionuclidic therapies are not restricted to the previously mentioned examples. Multiple other indications are currently being explored including tumors other than NET that express SSTR (like breast, small-cell lung cancer, pheochromocytoma, and meningioma); as well as other PSMA-expressing tumors like hepatocellular carcinoma and renal cell carcinoma. On the other hand, other molecular targets are being investigated like fibroblast activation protein (FAP) which is up- regulated by cancer-associated fibroblasts, among many other potential targets.
In summary, radiotheranostics is still in its early stages and interdisciplinary efforts are needed to overcome various challenges. Moving forward, the main goal is to integrate radiotheranostics with other cancer therapies like chemotherapy and immune modulation to support precise and personalized cancer therapy in both palliative and curative settings.